Hbro3 Strong or Weak Acid or Base
Classify the compounds as a strong acid weak acid strong base or weak base. Hydrofluoric Acid HF weak acid.
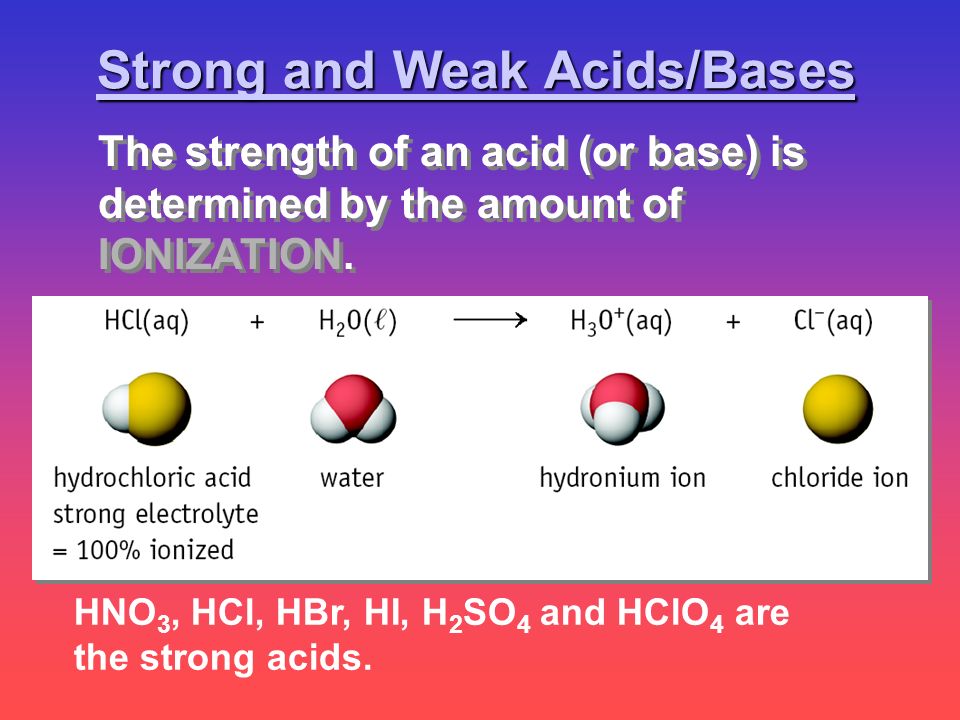
Hno 3 Hcl Hbr Hi H 2 So 4 And Hclo 4 Are The Strong Acids Strong And Weak Acids Bases The Strength Of An Acid Or Base Is Determined By The Amount Ppt Download
The pKa of HBrO3 is -2 and of HBr04 is -46.

. Hypobromous acid is a weak unstable acid with the chemical formula HBrO where the bromine atom is in the 1 oxidation state. Bromic acid formula HBrO3. It may be 1 ionized or 99 ionized but it is still classified as a weak acid.
Bromic acid HBrO3 or BrHO3 CID 24445 - structure chemical names physical and chemical properties classification patents literature biological activities. Uniform Reactions Are Not Probable Taking two different test subjects and stating what their reactions will be to similar situations is not possible. Bromic acid also known as hydrogen bromate is an oxoacid with the molecular formula HBrO3.
Because oxygen is a highly electronegative atom it pulls. H3PO4 Phosphoric acid is a weak acid due to the fact that it does not fully dissociate in water. Is HBR a strong acid or a strong base.
In chemistry neutralization or neutralisation see spelling differences is a chemical reaction in which an acid and a base react quantitatively with each other. Classify the compounds as a strong acid weak acid strong base or weak base. Hbro3 strong or weak acid or base.
Sulfuric Acid H2SO4 strong acid. Acetic Acid HC2H3O2 weak acid. Start studying Strong and Weak Acids and Bases.
This means that both are strong acids completely ionized in water. It has an H 0 value of 151 compared to 12 for sulfuric acid. HBrO4 is a stronger acid than HBrO3 by.
A very strong base always forms a weak conjugate acid. These acids all fall into the category of superacids acids stronger than 100 sulfuric acid. Is HIO3 an acid or base.
A strong acid B weak acid C impossible to tell 6. Related guide for Is HBrO3 Weak Or Strong. HBrO3 only exists in solution but is a strong acid so its completely dissociated into Haq and BrO3-aq.
Chloric acid formula HClO3. If you dissolve 0025 mol of HBr in 1 L of water the resulting solution contains 0025 mol of H30. Is sulphurous acid an acid or base.
The concept of conjugate acid-base pair. HBraq OH-aq H201 Braq A HBO B OH C H20 D Br 5. It is weak acid.
HBr is a strong acid. HSO 3 F is one of the strongest known simple Brønsted acids although carborane-based acids are still stronger. Is H3PO4 a strong acid or weak acid.
Hydroiodic acid formula HI. It is a bromine oxoanion and a monovalent inorganic anion. HBrO3 is a stronger acid than HBrO2 because it has more oxygens surrounding the central Br atom.
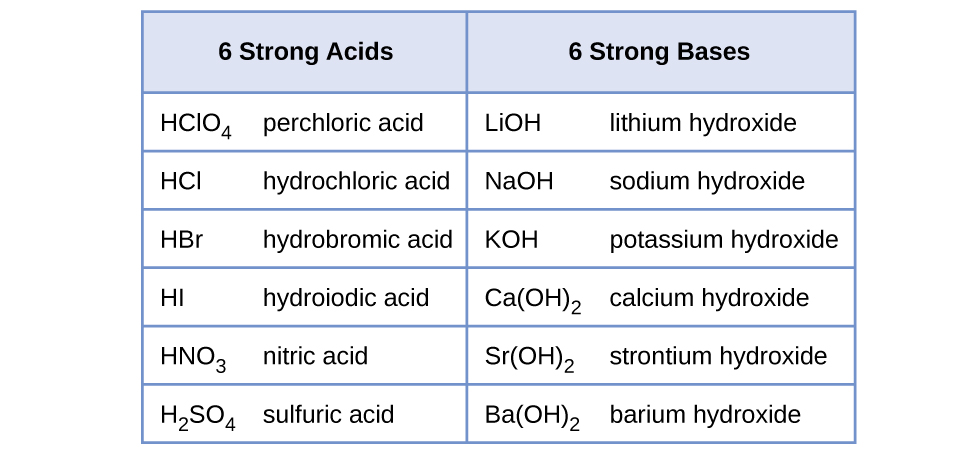
HF Hydrofluoric acid is a weak acid but you cant store it in glass bottles as it will dissolve the glass. Is HBrO a strong acid. LIST ACID NH4ClO4 NH4Cl HBrO WEAK H2PO4-H3PO3 WEAK HNO3 STRONG HCl STRONG H2S WEAK H2SO4 STRONG H3PO4 WEAK H2CO3 WEAK HBr STRONG HI STRONG HClO4 STRONG HClO3.
Chlorous acid formula HClO2. Hydrobromic acid formula HBr. Chemistry questions and answers.
Identify the conjugate base in the following reaction. Consequently is NaOBr an acid or base. Acid Strength and Bond Strength.
Lets put some numbers on this. Because it is a weak acid its conjugate base OBr- when in a salt form with a metal such as Na giving NaOBr which is a weak base and would produce an alkaline basic solution in water. So no since HBrO3 and HBrO4 dont completely dissociate they are not considered strong acids.
Strong acid Weak acid Strong base Weak base Answer. So no since HBrO3 and HBrO4 dont completely dissociate they are not considered strong acids. A very strong acid always forms a weak conjugate base.
Among these three acids HClO3 is the. It is a conjugate base of a hypobromous acid. Formic Acid HCHO2 weak acid.
Strong acid Weak acid Strong base Weak base Answer Bank H2CO3 KOH NH3 HBr H3PO4 HSO4 Ba OH2. Which is the strongest acid HClO4 or HBrO3. Yes it is a salt but when hydolyzed in water it will have a pH that is slightly basic.
A very weak acid always forms a strong conjugate base. As you see in the above reaction NH3 is a weak base and we know a weak base always forms a conjugate acid not necessarily the strong one. H3AsO4 is a stronger acid than H3PO4 because As is larger than P.
So obviously its conjugate acid-base. Perchloric acid formula HClO4. HIO3 Is Generally Considered A Weak Acid When The Solvent Is Water But Is A Strong Acid When Dissolved In Acetic Acid.
Is phosphoric acid strong or weak acid. Is HBrO3 acid or base. A very weak base always forms a strong conjugate acid.
Why is HIO3 a strong acid. Is bromate harmful to humans. Learn vocabulary terms and more with flashcards games and other study tools.
As we know HNO 3 sometimes acts as the base and sometimes as an acid. Learn vocabulary terms and more with flashcards games and other study tools. Start studying Strong and weak acids and bases.
Click to see full answer. If it is less than 100 ionized in solution it is a weak baseLearning Objectives. This is due to a weak covalent bond between H and Br atoms in the molecule giving a strong ionic characteristic to the bonding.
As we discussed earlier NH 3 is a weak base hence it will form a conjugate acid by adding one proton to itself. A strong base is a base that is 100 ionized in solution. Based on this information is HBr a strong acid or weak acid.
Is HBr weak or strong acid. Is HF a strong acid. Hydrochloric acid formula HCl.
Cancer Hazard Potassium Bromate may be a CARCINOGEN in humans since it has been shown to cause kidney thyroid and gastrointestinal cancer in animals. Ability to Categorize Observable Behaviors When studying the repeated actions of the individuals over prolonged periods of time common actions were noticed. Its pKa value is -9 and therefore it almost completely ionises to give a proton in aqueous form.
Because it is formed from the reaction of a strong base MgOH2 and a. The issue is similar with bases. HCl HBr and HI are all strong acids whereas HF is a weak acid.
Hypochlorous acid formula HClO.

Some Examples For Strong And Weak Acids And Bases

How To Determine If Acid Is Strong Or Weak Shortcut W Examples And Practice Problems Youtube
Comments
Post a Comment